You are here: Home: PCU 1|2005: Daniel P Petrylak, MD
| Daniel P Petrylak, MD |
EDITED COMMENTS |
 Recent Phase III trials evaluating docetaxel-based combinations in patients with hormone-insensitive metastatic disease Recent Phase III trials evaluating docetaxel-based combinations in patients with hormone-insensitive metastatic disease
Our first studies evaluating docetaxel plus estramustine were performed in the laboratory in 1995. We were excited by what we saw in vitro and moved forward into a Phase I study that opened in February of 1996.
One of the old jokes about Phase I studies is that the first patient responds but then nobody else does. Well, the opposite happened in that study: The first patient didn’t respond, but nearly every subsequent patient did. We saw promising responses in patients who were heavily pretreated. Median survival was close to 24 months, and that was the highest reported median survival of any study at that time.
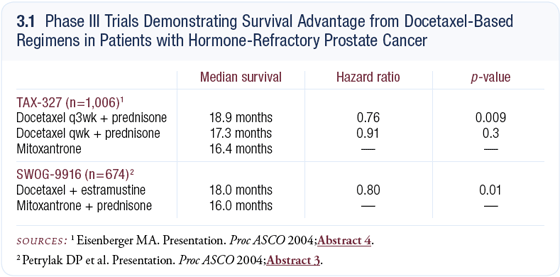
This background provided the basis for SWOG-9916 (Petrylak 2004a, b), which is a randomized trial comparing docetaxel/estramustine to mitoxantrone/prednisone in men with progressive androgen-independent prostate cancer and softtissue or bony metastases. These were not the asymptomatic patients with rising PSA only. They had to progress by one of three criteria: bone scan, CT or PSA. The trial opened in October 1999 and closed in January 2003. We demonstrated a 20 percent reduction in the rate of death in favor of those patients who received docetaxel/estramustine; however, estramustine-related toxicity was problematic and included deep venous thromboses, cardiovascular events and nausea.
A related and important trial was TAX-327 (Eisenberger 2004), which compared docetaxel weekly or every three weeks plus prednisone to mitoxantrone/ prednisone. Survival was improved with every three-week docetaxel. The data from both studies demonstrate for the first time that we have a chemotherapeutic agent — docetaxel — that results in prolonged survival for men with hormonerefractory prostate cancer (3.1).
Because the estramustine-related toxicity was problematic and the median survival and hazard ratios are similar for docetaxel/prednisone and docetaxel/ estramustine, the FDA has recommended docetaxel/prednisone as the standard of care for hormone-refractory metastatic prostate cancer.
Nonprotocol therapy for patients with hormone-refractory metastatic disease
The FDA approved docetaxel for patients with hormone-refractory metastatic prostate cancer, but they didn’t specify when it should be utilized. Hormone-refractory prostate cancer is a continuum. In general, the first sign of disease breakthrough is a rising PSA, and the patient is often asymptomatic. Generally, after seven to 12 months, we start seeing changes in scans, and patients become symptomatic. A window exists during which markers are going up and the patient is asymptomatic, yet the patient may want treatment.
Often physicians will try a second hormonal manipulation, such as Nizoral®, high-dose bicalutamide or nilutamide. All of these seem to have a 20 percent to 40 percent rate of response and a median time to progression of about four months, but no proven survival benefit.
An interesting observation gleaned from a subanalysis of TAX-327 data is that the hazard ratios for survival are similar whether patients are asymptomatic or symptomatic, and the difference of two months in median survival is conserved for both symptomatic and asymptomatic patients.
It is difficult to decide whether to utilize docetaxel in patients who are asymptomatic but have rising PSAs. It is important to evaluate how rapidly the disease is progressing. Clearly, if the PSA is not rising rapidly, you have time to try other manipulations. In my experience, by the time those manipulations fail, patients need chemotherapy.
In asymptomatic patients with rapidly rising or rapidly doubling PSA levels, progression of soft-tissue disease or progression on bone scan, I consider initiating chemotherapy. During the initial PSA rise, unless the patient has visceral disease, I’m not in favor of using chemotherapy. I would utilize an investigational agent or a secondary hormonal manipulation.
To use a baseball analogy, docetaxel can be saved as the “relief pitcher” for late innings, or you can use it earlier as your starting pitcher. Either way, we know that docetaxel has a high response rate and a proven survival benefit.
Docetaxel-associated symptom improvement
In TAX-327, an improvement in quality of life occurred in patients receiving docetaxel compared to those receiving mitoxantrone/prednisone. Patients who are symptomatic from their disease often feel better, and we’ve seen dramatic improvements in symptoms such as bone pain.
We usually can control most of the side effects of chemotherapy, and patients are able to maintain a reasonable quality of life, continue to work and remain active in other areas of their lives. I tell patients that they have a 50/50 chance their symptoms will improve with docetaxel, but a flare may occur about a week after treatment is started.
Proposed CALGB trial evaluating docetaxel plus bevacizumab
The CALGB is planning to do a randomized trial of docetaxel with or without bevacizumab, which is a VEGF-inhibitor. An elevated serum level of vascular endothelial growth factor has been identified as an important prognostic factor in hormone-refractory prostate cancer, so it makes sense that using an antibody targeted against that particular target is effective.
We’re in the process of designing a Phase I study using docetaxel plus bevacizumab plus an anti-EGFR agent. In renal cell cancer, that approach — combining bevacizumab plus erlotinib — has had promising results.
Earlier integration of medical oncologists in management of prostate cancer
In the community, urologists usually attempt a couple of hormonal manipulations and then send their patients to the oncologist. The optimal time to start chemotherapy is a bit of an art, and no FDA guidelines delineate the proper time to start chemotherapy.
Not all patients with hormone refractory disease should start chemotherapy. I believe patients should see an oncologist initially, but they should never lose contact with their urologist. The urologist is the primary caregiver who diagnoses the disease and may have removed the prostate. These patients will continue to depend on their urologists for problems and complications that develop from the prostate cancer, such as urinary tract obstruction, stinting and transurethral resections of the prostate.
Defining the optimal time to initiate hormonal therapy
Randomized trial data suggest that earlier hormone therapy is beneficial at the point of PSA progression, but no data absolutely indicate benefit in the asymptomatic patient with a rising PSA. We know from studies of combination therapy that patients at high risk will benefit from early hormonal therapy plus radiation therapy. Ed Messing’s trial randomly assigned patients who had positive lymph nodes after prostatectomy to immediate hormonal therapy versus delayed hormonal therapy. The trial demonstrated that earlier hormonal therapy was beneficial (Messing 1999).
A number of important questions must be answered. Does a threshold value of PSA need to be defined for these patients? Does PSA doubling time depend on regional clinical characteristics? We need to investigate these questions.
Bone complications from hormonal therapy
Many patients receive androgen blockade at some point and face a whole new set of complications. Matt Smith presented interesting data at ASCO 2004, evaluating the Medicare database and analyzing patients with nonmetastatic disease who did or did not receive hormone therapy.
The single most important prognostic factor for the development of osteoporosis in these patients was whether they had androgen blockade. The rate of fractures was proportional to the duration of androgen blockade (Smith 2004). Other side effects include muscle wasting, loss of energy and diminished sexual function.
Use of maximal androgen blockade
The survival data from the SWOG studies — particularly SWOG-8494, in which Dave Crawford was the principal investigator — showed approximately a three-month improvement in survival in favor of combined blockade compared to an LHRH agonist alone (Crawford 1989, 1990).
I use maximal androgen blockade. Certainly, we’ve treated patients with more aggressive therapy for less of a survival benefit. I believe it can’t hurt. And if it can’t hurt and has a possibility to improve survival, I will use the combined blockade with bicalutamide, which is the easiest drug for me to administer and for the patient to receive.
Select publications
 |
| Dr Petrylak is an Associate Professor of Medicine and Director of the Genitourinary Oncology Program at Columbia Presbyterian Medical Center in New York, New York. |
|

