Select Excerpts from the Discussion
CD 2, Track 15
 DR LOVE: Could you comment on the combination of high-dose radiation
therapy and androgen deprivation for patients at high risk?
DR LOVE: Could you comment on the combination of high-dose radiation
therapy and androgen deprivation for patients at high risk?
 DR ZELEFSKY: Dose response data for patients at high risk suggest that a
significant percentage of these patients may have localized disease warranting
aggressive local therapy. However, it is unclear if a patient with low-volume,
high-grade disease needs prolonged hormonal therapy or whether an aggressive
course of local therapy is sufficient.
DR ZELEFSKY: Dose response data for patients at high risk suggest that a
significant percentage of these patients may have localized disease warranting
aggressive local therapy. However, it is unclear if a patient with low-volume,
high-grade disease needs prolonged hormonal therapy or whether an aggressive
course of local therapy is sufficient.
Currently, with IMRT conformal treatments, there’s a slight increase in
urinary side effects and chronic obstructive symptoms with the higher dose, but this is not that significant clinically. The rectal bleeding rates have been
only three percent despite applications as high as 86 Gray. We’re examining
the impact of the higher doses on quality of life, but I don’t believe they make
a tremendous difference in regard to toxicity.
 DR KEANE: We know that 70 Gray combined with hormonal therapy works
far better than 70 Gray alone.
DR KEANE: We know that 70 Gray combined with hormonal therapy works
far better than 70 Gray alone.
The concern is that in the trials that are moving the radiation dose to 78 Gray,
we have no idea how that dose combined with hormonal therapy will affect
the patient in terms of toxicity. We know that increasing the radiation dose
results in more toxicity.
 DR CRAWFORD: Mike and others have clearly shown that escalating the dose
of radiation increases the response rate with minimal side effects. However,
I’m concerned about what we will see in these patients a decade later. We
know that radiation therapy can have long-term sequelae.
DR CRAWFORD: Mike and others have clearly shown that escalating the dose
of radiation increases the response rate with minimal side effects. However,
I’m concerned about what we will see in these patients a decade later. We
know that radiation therapy can have long-term sequelae.
 DR LOVE: Mike, what long-term toxicity data do we have with high-dose
radiation therapy?
DR LOVE: Mike, what long-term toxicity data do we have with high-dose
radiation therapy?
 DR ZELEFSKY: We now have data past 10 years, at least for 75 Gray of
conformal radiation therapy, and we have not seen hemorrhagic cystitis, rectal
strictures or the like.
DR ZELEFSKY: We now have data past 10 years, at least for 75 Gray of
conformal radiation therapy, and we have not seen hemorrhagic cystitis, rectal
strictures or the like.
The risk of developing secondary cancers is greater in these patients than in
the general population, and they need to be followed. I agree that as we move
into these uncharted trials of higher radiation doses, we need to look at the
long-term toxicities in 10 and 15 years.
CD 2, Track 17
 DR LOVE: Laurie, how do you decide on the duration of hormonal
therapy with radiation treatment, and does it change based on the
patient’s age?
DR LOVE: Laurie, how do you decide on the duration of hormonal
therapy with radiation treatment, and does it change based on the
patient’s age?
 DR KLOTZ: The data are not that clear as to the optimal duration. In my
practice, I use six months to three years of therapy, and I titrate it according
to the patient’s risk and the degree to which the patient tolerates hormonal
therapy.
DR KLOTZ: The data are not that clear as to the optimal duration. In my
practice, I use six months to three years of therapy, and I titrate it according
to the patient’s risk and the degree to which the patient tolerates hormonal
therapy.
I believe six months of hormonal therapy is a bare minimum with these
patients. In the face of the D’Amico study, the disease-free survival rate
improvement was the same with six months of therapy as in other studies of
two years in an intermediate-risk cohort (D’Amico 2004).
I’m not aware of any comparison of six months to longer durations in patients
at higher risk, but the argument to go beyond six months is not so compelling
that I would do so in a patient who is experiencing difficulty with the therapy.
 DR ZELEFSKY: Although the D’Amico study never compared the two
durations, the paper suggests that longer courses may be better. However,
lower doses of radiation were used, so the role of higher doses remains unclear.
Also, the way “high risk” has been described in trials is variable. Obviously,
the volume of disease is a critical aspect, and maybe it should be based on
volume rather than on these arbitrary nomenclatures of high risk.
DR ZELEFSKY: Although the D’Amico study never compared the two
durations, the paper suggests that longer courses may be better. However,
lower doses of radiation were used, so the role of higher doses remains unclear.
Also, the way “high risk” has been described in trials is variable. Obviously,
the volume of disease is a critical aspect, and maybe it should be based on
volume rather than on these arbitrary nomenclatures of high risk.
 DR KEANE: The Hanks data compared goserelin and flutamide two months
before and two months during radiation therapy with or without an additional
24 months of goserelin. In a subset analysis, the 28 months of therapy resulted in
significantly better overall survival in the so-called patients at high risk (Hanks
2003). I believe that’s the only hard evidence we have one way or the other.
DR KEANE: The Hanks data compared goserelin and flutamide two months
before and two months during radiation therapy with or without an additional
24 months of goserelin. In a subset analysis, the 28 months of therapy resulted in
significantly better overall survival in the so-called patients at high risk (Hanks
2003). I believe that’s the only hard evidence we have one way or the other.
 DR CHODAK: There are a lot of good randomized trials, but I’m concerned
that none of the studies is pure. Maybe you could argue that D’Amico’s study
is the best in the sense that there was a more limited group of intermediate-risk
patients, but even there, patients could enroll with a high PSA or with a
high Gleason score.
DR CHODAK: There are a lot of good randomized trials, but I’m concerned
that none of the studies is pure. Maybe you could argue that D’Amico’s study
is the best in the sense that there was a more limited group of intermediate-risk
patients, but even there, patients could enroll with a high PSA or with a
high Gleason score.
The lack of the homogeneity may, in part, explain why it takes 10 years to
see a benefit in one RTOG trial, whereas we saw a benefit earlier in the Bolla
trial (Bolla 2002). We may need to be more careful designing these trials.
We want to get them done and include a lot of patients at high risk, but we
may be camouflaging patients who actually have metastatic disease and would
benefit from hormone therapy versus patients with extensive local disease, who
would benefit from a higher dose of radiation.
CD 2, Track 18
 DR LOVE: Len, do you feel the multimodality approach is appropriate
when treating patients with high-risk disease after a prostatectomy?
DR LOVE: Len, do you feel the multimodality approach is appropriate
when treating patients with high-risk disease after a prostatectomy?
 DR GOMELLA: We know that treatment outcomes relate to risk stratification
and that patients at low risk have similar outcomes with any monotherapy and
patients at high risk generally do not do well with standard monotherapy. The
majority of patients who progress after therapy do have high-risk disease, and
we know that high-risk disease accounts for most of the prostate cancer deaths.
DR GOMELLA: We know that treatment outcomes relate to risk stratification
and that patients at low risk have similar outcomes with any monotherapy and
patients at high risk generally do not do well with standard monotherapy. The
majority of patients who progress after therapy do have high-risk disease, and
we know that high-risk disease accounts for most of the prostate cancer deaths.
What the best treatment is for patients at high risk is unclear, and we have
limited data from clinical trials for this group of patients, so it’s reasonable for
us to be evaluating multimodality approaches for these patients.
In clinical practice, it’s important for us to risk stratify each case, evaluate
the postoperative data and discuss the options with our patients. One should
consider adjuvant hormone therapy for patients with positive lymph nodes, based on the Messing study. One might question whether this could be the
new standard of care for patients at high risk (Messing 2006).
In addition, we should attempt to place patients on randomized clinical trials
because we really have a paucity of published data in this area.
 DR LOVE: Steve, how do you approach the patient who has high-risk disease
after radical prostatectomy?
DR LOVE: Steve, how do you approach the patient who has high-risk disease
after radical prostatectomy?
 DR FREEDLAND: I question whether it makes a difference if we treat the patient
immediately versus monitoring the patient and, if he recurs, gaining more information
— such as the PSA kinetics, the doubling time, the time to recurrence
— to better risk stratify that patient. I believe, based on my view of the literature,
that in most of these patients I have not lost anything by waiting.
DR FREEDLAND: I question whether it makes a difference if we treat the patient
immediately versus monitoring the patient and, if he recurs, gaining more information
— such as the PSA kinetics, the doubling time, the time to recurrence
— to better risk stratify that patient. I believe, based on my view of the literature,
that in most of these patients I have not lost anything by waiting.
I counsel my patients on the options, but I prefer the salvage route versus
adjuvant therapy. Delaying treatment allows patients to recover their potency,
their continence and the like.
In addition, a significant portion of men at high risk will not recur. Even in
margin-positive disease, only 50 percent of those patients recur, so we can avoid
therapy for some of those patients.
 DR LOVE: How do you approach the patient with node-positive disease?
DR LOVE: How do you approach the patient with node-positive disease?
 DR FREEDLAND: The Messing data show that if you treat now versus delaying
until the time of metastatic disease, you’re going to improve survival (Messing
2006).
DR FREEDLAND: The Messing data show that if you treat now versus delaying
until the time of metastatic disease, you’re going to improve survival (Messing
2006).
However, although I believe the Messing data from the therapeutic arm, the
control arm is not representative of my practice, so it’s hard to say that they are
meaningful data to me.
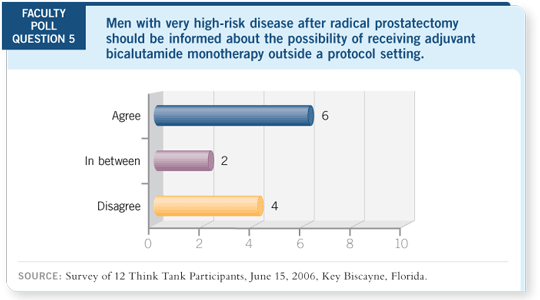
I would treat those patients once they recur, and the majority will recur with
positive nodes, but not 100 percent. Even in the control arm, there were some
men who were disease free for a long time. I believe you can avoid therapy in
some men and treat those who really need it early enough so that you haven’t
lost anything.
CD 2, Tracks 19-20
 DR LOVE: Paul, can you comment on the issue of treating the unusual
patient with node-positive disease?
DR LOVE: Paul, can you comment on the issue of treating the unusual
patient with node-positive disease?
 DR SCHELLHAMMER: I feel strongly that patients with node-positive disease
have a high enough risk and the data are strong enough to initiate androgen
deprivation therapy.
DR SCHELLHAMMER: I feel strongly that patients with node-positive disease
have a high enough risk and the data are strong enough to initiate androgen
deprivation therapy.
Patients at high risk, in general, are clinical trial candidates. However, if I
have a patient who is not on a clinical trial and whom I’m going to follow
with observation, my inclination is to get ultrasensitive PSAs from the get-go.
Then I can determine the rising PSA before it gets to the 0.2 level that we
have said is the beginning of failure. To me, that’s the absolute indication to
initiate another therapeutic option.
 DR ZIPPE: I prefer to wait and watch for elevation of the PSA. I am a big fan of
bicalutamide monotherapy. I look to treat with minimal side effects. I have patients
now who are six and seven years out, and their disease is still undetectable.
DR ZIPPE: I prefer to wait and watch for elevation of the PSA. I am a big fan of
bicalutamide monotherapy. I look to treat with minimal side effects. I have patients
now who are six and seven years out, and their disease is still undetectable.
 DR KEANE: I believe we need to reconsider this high-risk situation and decide
whether we are talking about palliation or cure. Examining the data from
Bolla, Hanks, Messing and the RTOG-8531, I believe we can cure some of these patients (Bolla 2002; Hanks 2003; Messing 2006; Lawton 2005). When
you look at the long-term data and the survival curves, after approximately
seven to 10 years they begin to closely mirror the general population.
DR KEANE: I believe we need to reconsider this high-risk situation and decide
whether we are talking about palliation or cure. Examining the data from
Bolla, Hanks, Messing and the RTOG-8531, I believe we can cure some of these patients (Bolla 2002; Hanks 2003; Messing 2006; Lawton 2005). When
you look at the long-term data and the survival curves, after approximately
seven to 10 years they begin to closely mirror the general population.
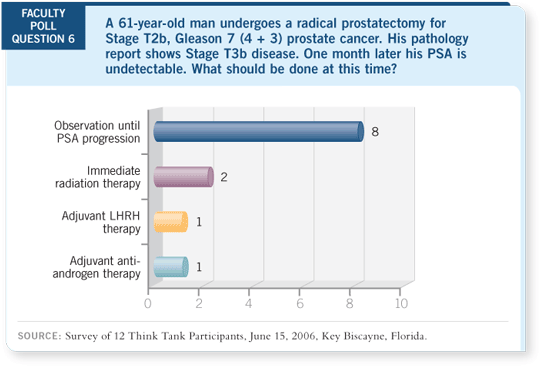
 DR FREEDLAND: If you think it is curative, if you have a patient with positive
nodes, you treat him with adjuvant androgen deprivation and his PSA remains
undetectable, do you stop hormonal therapy at some point and see what
happens?
DR FREEDLAND: If you think it is curative, if you have a patient with positive
nodes, you treat him with adjuvant androgen deprivation and his PSA remains
undetectable, do you stop hormonal therapy at some point and see what
happens?
 DR KEANE: Absolutely. After five years we’re measuring the testosterone
levels and discontinuing the anti-androgens and the LHRH agonists because
there must be a time point where you’ve burned out the hypothalamic
pituitary axis.
DR KEANE: Absolutely. After five years we’re measuring the testosterone
levels and discontinuing the anti-androgens and the LHRH agonists because
there must be a time point where you’ve burned out the hypothalamic
pituitary axis.
The Bolla trial gave us the first inclination. I believe 25 or 30 percent of the
patients never regained a normal testosterone level. That being the case, you
may not improve the patients’ side effects, because they’ll still have the side
effects of castration, but you save a considerable amount of money by discontinuing
treatment.
 DR FREEDLAND: If the patient’s testosterone returns to normal, do you restart
the therapy then?
DR FREEDLAND: If the patient’s testosterone returns to normal, do you restart
the therapy then?
 DR KEANE: I would.
DR KEANE: I would.
 DR FREEDLAND: So you’re not actually taking them off androgen deprivation,
correct?
DR FREEDLAND: So you’re not actually taking them off androgen deprivation,
correct?
 DR KEANE: Correct.
DR KEANE: Correct.
 DR FREEDLAND: If the idea is that it’s curative, there should be a point at
which you’ve reached the cure and you could stop therapy, allow their testosterone
to return to normal, and they should be fine.
DR FREEDLAND: If the idea is that it’s curative, there should be a point at
which you’ve reached the cure and you could stop therapy, allow their testosterone
to return to normal, and they should be fine.
 DR KEANE: In theory, yes.
DR KEANE: In theory, yes.
 DR LOVE: Unless you define it as a functional cure, which is that they have
the same life expectancy as an age-matched population. They may still have the
tumor present, it might still need to be suppressed, but they’re cured, clinically.
DR LOVE: Unless you define it as a functional cure, which is that they have
the same life expectancy as an age-matched population. They may still have the
tumor present, it might still need to be suppressed, but they’re cured, clinically.
 DR FREEDLAND: I would agree with that.
DR FREEDLAND: I would agree with that.
 DR CRAWFORD: We published a paper a few years ago looking at the 10-year survival rates in metastatic cancer, and approximately eight to 10 percent
of these people are alive at 10 plus years (Tangen 2003). In a lot of cancers,
we call that a cure. So I agree that hormone therapy does cure some patients
with prostate cancer. I don’t think there is any question about it when evaluating
the 13-year data we have right now. I mean, how long do we have to go
before we call it a cure?
DR CRAWFORD: We published a paper a few years ago looking at the 10-year survival rates in metastatic cancer, and approximately eight to 10 percent
of these people are alive at 10 plus years (Tangen 2003). In a lot of cancers,
we call that a cure. So I agree that hormone therapy does cure some patients
with prostate cancer. I don’t think there is any question about it when evaluating
the 13-year data we have right now. I mean, how long do we have to go
before we call it a cure?

CD 3, Tracks 2-4
 DR LOVE: Dave, can you discuss the role of adjuvant as opposed to
salvage radiation therapy after radical prostatectomy?
DR LOVE: Dave, can you discuss the role of adjuvant as opposed to
salvage radiation therapy after radical prostatectomy?
 DR CRAWFORD: With adjuvant radiation therapy, you have pros and cons. The
pros are better biochemical control, better local control, potentially lower doses
used and smaller tumors treated. The cons are no survival benefit has yet been
demonstrated, treating many patients who don’t need it, a lack of benefit if the
patient has metastatic disease, increased cost and risk of unnecessary toxicity.
DR CRAWFORD: With adjuvant radiation therapy, you have pros and cons. The
pros are better biochemical control, better local control, potentially lower doses
used and smaller tumors treated. The cons are no survival benefit has yet been
demonstrated, treating many patients who don’t need it, a lack of benefit if the
patient has metastatic disease, increased cost and risk of unnecessary toxicity.
With salvage radiation therapy, the pros and cons are a little different. The
pros are only treating those patients who are failing and increased cost effectiveness.
The cons are delaying therapy, no benefits with metastases and
treating larger tumors that are maybe less responsive.
 DR LOVE: Steve, how do you approach the consideration of postprostatectomy
radiation therapy?
DR LOVE: Steve, how do you approach the consideration of postprostatectomy
radiation therapy?
 DR FREEDLAND: According to the data published in The Lancet from
EORTC-22911, about 50 percent of the patients with positive margins who
were not treated with adjuvant radiation therapy had a PSA recurrence. Of those patients with positive margins who were treated with adjuvant radiation
therapy, about 70 percent did not have a PSA recurrence (Bolla 2005).
DR FREEDLAND: According to the data published in The Lancet from
EORTC-22911, about 50 percent of the patients with positive margins who
were not treated with adjuvant radiation therapy had a PSA recurrence. Of those patients with positive margins who were treated with adjuvant radiation
therapy, about 70 percent did not have a PSA recurrence (Bolla 2005).
In Stephenson’s data, there was about a 65 percent response to salvage radiation
therapy among the select group of men who were eligible (Stephenson
2004). I think putting these two studies’ results together makes a good
argument for salvage radiation therapy. You are not treating the men who do
not need it, and in the men who do need it, you are able to provide them an
almost equally efficacious therapy at the time of recurrence.
 DR ZELEFSKY: The data from SWOG-S8794 are important, but they do not
yet demonstrate that if we followed patients carefully and on a detectable and
bona fide rise in PSA, we could obtain the same disease-free survival benefit
with radiation therapy. The interesting thing about the SWOG-S8794 data is
that there was a crossover of patients, and it perhaps shows that earlier administration
of radiation therapy compared to later will benefit patients.
DR ZELEFSKY: The data from SWOG-S8794 are important, but they do not
yet demonstrate that if we followed patients carefully and on a detectable and
bona fide rise in PSA, we could obtain the same disease-free survival benefit
with radiation therapy. The interesting thing about the SWOG-S8794 data is
that there was a crossover of patients, and it perhaps shows that earlier administration
of radiation therapy compared to later will benefit patients.
Our practice, in general, even for a patient with a positive margin, is to discuss
the options of adjuvant versus salvage radiation therapy. More often than not,
we would recommend salvage radiation therapy on manifestation of a detectable
and rising PSA.
 DR CRAWFORD: I agree with Michael. In my practice I wait until the patient
has a biochemical failure. If they have a failure, I treat them early.
DR CRAWFORD: I agree with Michael. In my practice I wait until the patient
has a biochemical failure. If they have a failure, I treat them early.
 DR GOMELLA: With postoperative radiation therapy, you have to remember a
couple of things that may or may not be true. Number one, you do not radiate
everybody; you radiate people with a high risk of recurrence. You figure out
who is at risk for progression and then you offer them the radiation therapy.
DR GOMELLA: With postoperative radiation therapy, you have to remember a
couple of things that may or may not be true. Number one, you do not radiate
everybody; you radiate people with a high risk of recurrence. You figure out
who is at risk for progression and then you offer them the radiation therapy.
 DR LOVE: Lenny, can you describe a typical patient for whom you would use
postoperative radiation therapy?
DR LOVE: Lenny, can you describe a typical patient for whom you would use
postoperative radiation therapy?
 DR GOMELLA: We rely heavily on the Kattan nomogram (Stephenson 2005).
If they have less than a 25 percent risk of recurrence after radical prostatectomy,
based on their postoperative features, we mention it to them, but we do
not usually recommend it.
DR GOMELLA: We rely heavily on the Kattan nomogram (Stephenson 2005).
If they have less than a 25 percent risk of recurrence after radical prostatectomy,
based on their postoperative features, we mention it to them, but we do
not usually recommend it.
For patients with a greater than 50 percent risk, we strongly recommend they
consider adjuvant radiation therapy within three to six months of the radical
prostatectomy. In those patients with a risk that is somewhere in between, we
offer it as an option and let the patient decide. Ultimately the patients decide
across the board, but we strongly encourage the patients with a greater than 50
percent recurrence risk to think about adjuvant radiation therapy.
 DR LOVE: Mike, can you comment on the issue of morbidity?
DR LOVE: Mike, can you comment on the issue of morbidity?
 DR ZELEFSKY: In the shorter term or even in the midrange post-treatment,
we see exacerbation of stress incontinence. Increased numbers of bladder-neck
strictures occur down the road, and there is an increased risk of proctitis.
DR ZELEFSKY: In the shorter term or even in the midrange post-treatment,
we see exacerbation of stress incontinence. Increased numbers of bladder-neck
strictures occur down the road, and there is an increased risk of proctitis.
Although the risks of serious injuries with the proper techniques remain low,
it’s incumbent upon anybody discussing postoperative or salvage radiation
therapy to indicate that the morbidity will be increased above and beyond
radical prostatectomy. We are increasing potential complications, albeit small.
 DR KEANE: If you wait until the PSA starts to rise, are you going to do the
patient a disservice? Is the disease going to have any poorer outcome? Has
there ever been a head-to-head comparison of immediate versus delayed radiation
therapy?
DR KEANE: If you wait until the PSA starts to rise, are you going to do the
patient a disservice? Is the disease going to have any poorer outcome? Has
there ever been a head-to-head comparison of immediate versus delayed radiation
therapy?
 DR KLOTZ: A presentation at ASCO 2006 demonstrated that the response
to salvage radiation therapy improved according to the PSA prior to radiation
therapy, and the response was best when the PSA was below 0.2 ng/mL
(MacDonald 2006).
DR KLOTZ: A presentation at ASCO 2006 demonstrated that the response
to salvage radiation therapy improved according to the PSA prior to radiation
therapy, and the response was best when the PSA was below 0.2 ng/mL
(MacDonald 2006).
It is clear that patients do better the earlier they are treated, if they need
treatment. So pick the ones who are almost certainly going to progress and
treat them because there is no point in waiting. Then for the rest, I think it’s
reasonable to take the small risk and wait.

CD 3, Track 6
 DR LOVE: Mario, can you review the ongoing adjuvant chemotherapy
trials?
DR LOVE: Mario, can you review the ongoing adjuvant chemotherapy
trials?
 DR EISENBERGER: In SWOG-S9921, patients with high-risk disease are
randomly assigned following radical prostatectomy to receive immediate
hormonal therapy for two years with or without six months of mitoxantrone
and prednisone. This study started in 1999, which was prior to the taxane era.
The study is having difficulty in accrual, but I hope they will complete it. An
issue with the trial is that it does not have a no-treatment control arm.
DR EISENBERGER: In SWOG-S9921, patients with high-risk disease are
randomly assigned following radical prostatectomy to receive immediate
hormonal therapy for two years with or without six months of mitoxantrone
and prednisone. This study started in 1999, which was prior to the taxane era.
The study is having difficulty in accrual, but I hope they will complete it. An
issue with the trial is that it does not have a no-treatment control arm.
About three years ago, we conducted a pilot adjuvant feasibility trial evaluating
docetaxel (Rosenbaum 2006). Patients had their pathology reviewed
centrally at Johns Hopkins University, and we looked at the Rw model,
which separates patients according to their probability of relapse. The trial was
done before TAX-327 was reported (Tannock 2004), which is why we chose
weekly docetaxel.
When we then plugged it into Mike Kattan’s nomogram, we saw a shift
suggesting a better outcome than predicted, but obviously this is very preliminary.
The data, in addition to TAX-327, were what led us into the current
study design.
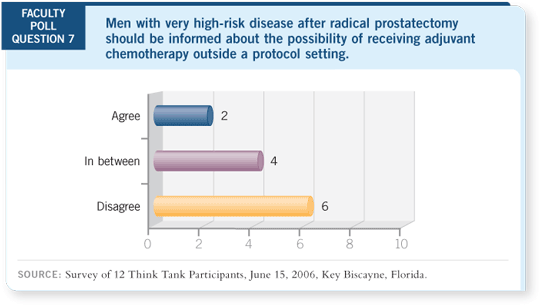
TAX-3501 includes patients who have no more than a 60 percent probability
of remaining disease free at five years by the Kattan nomogram (Stephenson
2005). Patients are randomly assigned to either observation with treatment
at the time of progression or immediate treatment. In both groups, patients
are randomly assigned to 18 months of leuprolide with or without docetaxel.
Essentially, this trial evaluates whether early treatment is more effective than
deferred treatment.
CD 3, Track 7
 DR PETRYLAK: I think we need to complete SWOG-S9921. If we change our
chemotherapy every time some new results come out, we’re never going to be
able to complete a randomized trial.
DR PETRYLAK: I think we need to complete SWOG-S9921. If we change our
chemotherapy every time some new results come out, we’re never going to be
able to complete a randomized trial.
 DR CRAWFORD: I agree. We need to complete these trials. We’re 20 years
behind other tumors. Regarding SWOG-S9921, docetaxel was around when
we designed the trial, but we thought we should use something that was better
tolerated. There were other tumors that were using less aggressive chemotherapy.
I think the downside of the trial is we don’t have a no-treatment
control arm.
DR CRAWFORD: I agree. We need to complete these trials. We’re 20 years
behind other tumors. Regarding SWOG-S9921, docetaxel was around when
we designed the trial, but we thought we should use something that was better
tolerated. There were other tumors that were using less aggressive chemotherapy.
I think the downside of the trial is we don’t have a no-treatment
control arm.
Select publications

